With the support of the Ministry of Science and Technology, the National Natural Science Foundation of China and the Chinese Academy of Sciences, the Organic Catalysis Task Force of the Key Laboratory of Molecular Recognition and Function Institute of the Institute of Chemistry has long been committed to the development of new asymmetric biomimetic primary amine catalytic systems. Recently, they have successfully overcome the challenging scientific problem of asymmetric enamine protonation through the "primary amine-tertiary amine" bifunctional synergistic catalyst, and achieved excellent catalytic activity and stereoselectivity. Related research results were published in "German Applied Chemistry" (Angew. Chem. Int. Ed., 2011, 50, 11451-11455).
The primary amine catalytic mechanism through the enamine mechanism is an important catalytic mode of enzymatic catalysis in nature, and is also a hot spot in the research of biomimetic catalysis in recent years. In the past few years, the research group designed and developed a new type of "primary amine-tertiary amine" bifunctional synergistic catalyst, and successfully applied to a series of substrate asymmetric aldol reaction research, achieved Efficient simulation of aldolase-like enzymes. Research shows that this type of catalyst is not only suitable for various ketones and aldehyde substrates (such as chain ketones, cyclic ketones, hydroxyacetone, dihydroxyacetone, pyruvate derivatives, ethyl acetoacetate derivatives, acetaldehyde, etc.) The asymmetric direct aldol reaction can also realize the asymmetric retro-aldol and transfer-aldol reactions of racemic aldol products. Relevant work has been published in "International Journal of American Chemistry", "European Chemistry", "Organic Chemistry Express" and other internationally renowned journals (J. Am. Chem. Soc. 2007, 129, 3074-3075; Org. Lett. 2008, 10, 653-656; Org. Lett. 2008, 10, 1775-1778; J. Org. Chem. 2009, 74, 1747-1750; J. Org. Chem. 2009, 74, 9521-9523; Chem. Eur. J. 2010, 16, 4457-4461; J. Org. Chem. 2010, 75, 4501–4507; Eur. J. Org. Chem. 2011, 3347–3352.) (Figure 1).
Further research found that covalently linking the primary amine catalyst to the cyclodextrin-type supramolecular host can achieve the supramolecular primary amine catalysis in the aqueous phase, and exhibit obvious enzyme kinetic behavior. This system is effectively grafted with small molecule catalysis and enzyme catalysis. It is the first asymmetric supramolecular catalysis system under pseudoenzyme conditions (J. Am. Chem. Soc. 2010, 132, 7216–7228). Detailed catalytic mechanism The research laid the foundation for the design and development of a new generation of biomimetic primary amine catalytic system. In addition, the covalent or non-covalent loading strategy can also improve the practicability of the biomimetic primary amine catalyst, and realize the efficient recycling of the catalyst (Chem. Eur. J. 2008, 14, 1273-1281; Chem. Commun 2008, 5719-5721; Eur. J. Org. Chem. 2009, 132-140; Eur. J. Org. Chem. 2009, 4486-4493; Chem. Commun., 2011, 47, 12325-12327) (Figure 2).
Asymmetric protonation is the most direct and atomically economical route for chiral synthesis of carbonyl compounds. It is a challenging research topic in the field of asymmetric catalysis; asymmetric protonation with enamine as an intermediate is a dream in this field Unresolved puzzles. On the basis of previous work, the research group further extended the biomimetic primary amine catalytic system to an asymmetric Fock-protonation reaction based on the imine-enamine mechanism. Unlike other previous reports, this reaction uses α-substituted acrolein substrate as the electrophile, and does not generate chirality in the CC bond formation step, but generates chiral centers during the protonation of enamine intermediates . The study found that the biomimetic primary amine catalytic system can effectively control the spatial orientation of the protonation process of enamine intermediates, and achieve effective stereo control of the smallest electrophiles in nature. The mechanism research shows that the real proton source in this reaction is the Water molecules generated or added in situ in the system. In the protonated transition state, the weak OH ... π interaction between the water molecule and the indole 4-position carbon plays an important role in the selectivity of the reaction (Angew. Chem. Int. Ed., 2011, 50, 11451 -11455) (Figure 3).
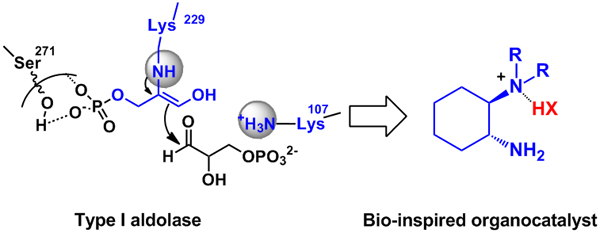
Figure 1 "Primary-tertiary amine" pseudoenzyme catalysis
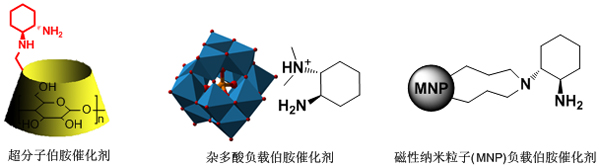
Figure 2 Supramolecular and supported primary amine catalyst
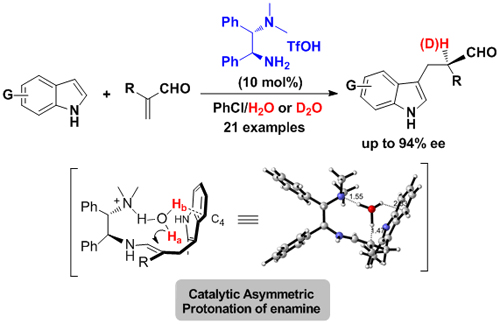
Figure 3 Asymmetric protonation catalyzed by "primary-tertiary amine" bifunctional catalyst
Jia Teng Glass hanging vase is widely exported to the world market. They're a Hanging Glass vase and they're perfect for floating air plants, ornaments, tiny garden scenes, or painted with a votive candle. You families will surely love them! It is made of borosilicate glass that is very clear and delicate. Borosilicate glass is a strong heat-resistant glass traditionally used to make scientific lab glass. It is simply a different type of glass that is resistant to temperature swings and scratching. Because the particles are held together so tightly, borosilicate glass is resistant to temperature swings. Also it is BPA free and no lead &mercury.
Hanging Glass Vase,Hanging Wall Glass Vase,Hanging Glass Terrarium,Hanging Glass
Hejian Jia Teng Glass Products Co., Ltd. , http://www.jtglassware.com